Advantages of Microneedles
Microneedles include solid needle, hollow needle, coated needle, soluble microneedle and swelling needle. Based on its own research and development practices, BIOQINGLAM is optimistic about the application of soluble microneedle technology in drug delivery and diagnosis. Microneedle drug delivery technology is a practical science and a results-oriented technology.
-
 Painless and non-invasive, patient compliance is the highest, user experience is similar to band-aids, no need for professionals' guidance or help, patients could operate it themselves.
Painless and non-invasive, patient compliance is the highest, user experience is similar to band-aids, no need for professionals' guidance or help, patients could operate it themselves. -
 The dosage is reduced to 1/5 to 1/200 of the dosage of traditional dosage forms ,As the dosage decreases sharply, side effects are negligible.
The dosage is reduced to 1/5 to 1/200 of the dosage of traditional dosage forms ,As the dosage decreases sharply, side effects are negligible. -
 The delivery of vaccine with microneedle patches has been proven to trigger an enhanced immune response and save dose. In addition, microneedle drug delivery has been shown to enhance the pharmacokinetic and pharmacodynamic effects of certain drugs.
The delivery of vaccine with microneedle patches has been proven to trigger an enhanced immune response and save dose. In addition, microneedle drug delivery has been shown to enhance the pharmacokinetic and pharmacodynamic effects of certain drugs. -
 Microneedling drug delivery is adequate for a wide range of active (therapeutic and vaccine) and inactive compounds, including small molecules, nucleic acids, peptides, proteins, and micro/nanoparticles.
Microneedling drug delivery is adequate for a wide range of active (therapeutic and vaccine) and inactive compounds, including small molecules, nucleic acids, peptides, proteins, and micro/nanoparticles. -
 The proprietary formulation and manufacturing process have proven that our microneedle patches maintain the stability of their pharmaceutical actives at room temperature, thereby reducing or so far as to eliminate the cold chain.
The proprietary formulation and manufacturing process have proven that our microneedle patches maintain the stability of their pharmaceutical actives at room temperature, thereby reducing or so far as to eliminate the cold chain.


Core Technology
1. Technology Leader
BIOQINGLAM is the technology leader in painless transdermal delivery of dissolving microneedles, with mature high-precision microneedle mold preparation technology, stable microneedle automation, and modular production processes. We have independently applied for eighteen patents, including twelve invention patents, one authorized patent, two PCT applications, three utility model patents, and three exterior design patents.
2. One-Stop Service for Preparation Development
BIOQINGLAM has a microneedle drug delivery R&D center of nearly 5,000 square meters, fully equipped with animal experiments, preparation development, process research, quality research, equipment development, quality system assurance, regulations, IT personnel; equipped with LC-MS, UPLC, HPLC, ELISA, CE, qPCR, SDS-PAGE, and other instruments; provide one-stop service for IND declaration including the conceptual study of pharmacodynamic pharmacokinetics, formulation development, production process development, quality research, and stability research.
3. cGMP Production Workshop and Automatic Mass Production
The cGMP workshop area for various types of drug-loaded microneedle patches that can meet sterility requirements is nearly 3,000 square meters. Microneedle patch preparation batches can be flexibly formulated and implemented in fully automated production, and the output reaches 4200 pieces per batch.
4. Diversity and Safety of Drug Delivery Routes
BIOQINGLAM already has an R&D pipeline of nucleic acid drugs, monoclonal antibodies, peptides, and chemical drugs. Moreover, the above pipelines have made breakthroughs to meet the research and development of various types of drugs. Customizable needle parameters enable painless drug delivery, and unique drug delivery routes push through dose limits for better safety.
BIOQINGLAM is the technology leader in painless transdermal delivery of dissolving microneedles, with mature high-precision microneedle mold preparation technology, stable microneedle automation, and modular production processes. We have independently applied for eighteen patents, including twelve invention patents, one authorized patent, two PCT applications, three utility model patents, and three exterior design patents.
2. One-Stop Service for Preparation Development
BIOQINGLAM has a microneedle drug delivery R&D center of nearly 5,000 square meters, fully equipped with animal experiments, preparation development, process research, quality research, equipment development, quality system assurance, regulations, IT personnel; equipped with LC-MS, UPLC, HPLC, ELISA, CE, qPCR, SDS-PAGE, and other instruments; provide one-stop service for IND declaration including the conceptual study of pharmacodynamic pharmacokinetics, formulation development, production process development, quality research, and stability research.
3. cGMP Production Workshop and Automatic Mass Production
The cGMP workshop area for various types of drug-loaded microneedle patches that can meet sterility requirements is nearly 3,000 square meters. Microneedle patch preparation batches can be flexibly formulated and implemented in fully automated production, and the output reaches 4200 pieces per batch.
4. Diversity and Safety of Drug Delivery Routes
BIOQINGLAM already has an R&D pipeline of nucleic acid drugs, monoclonal antibodies, peptides, and chemical drugs. Moreover, the above pipelines have made breakthroughs to meet the research and development of various types of drugs. Customizable needle parameters enable painless drug delivery, and unique drug delivery routes push through dose limits for better safety.
Principle of Microneedle Drug Delivery
Solid needle
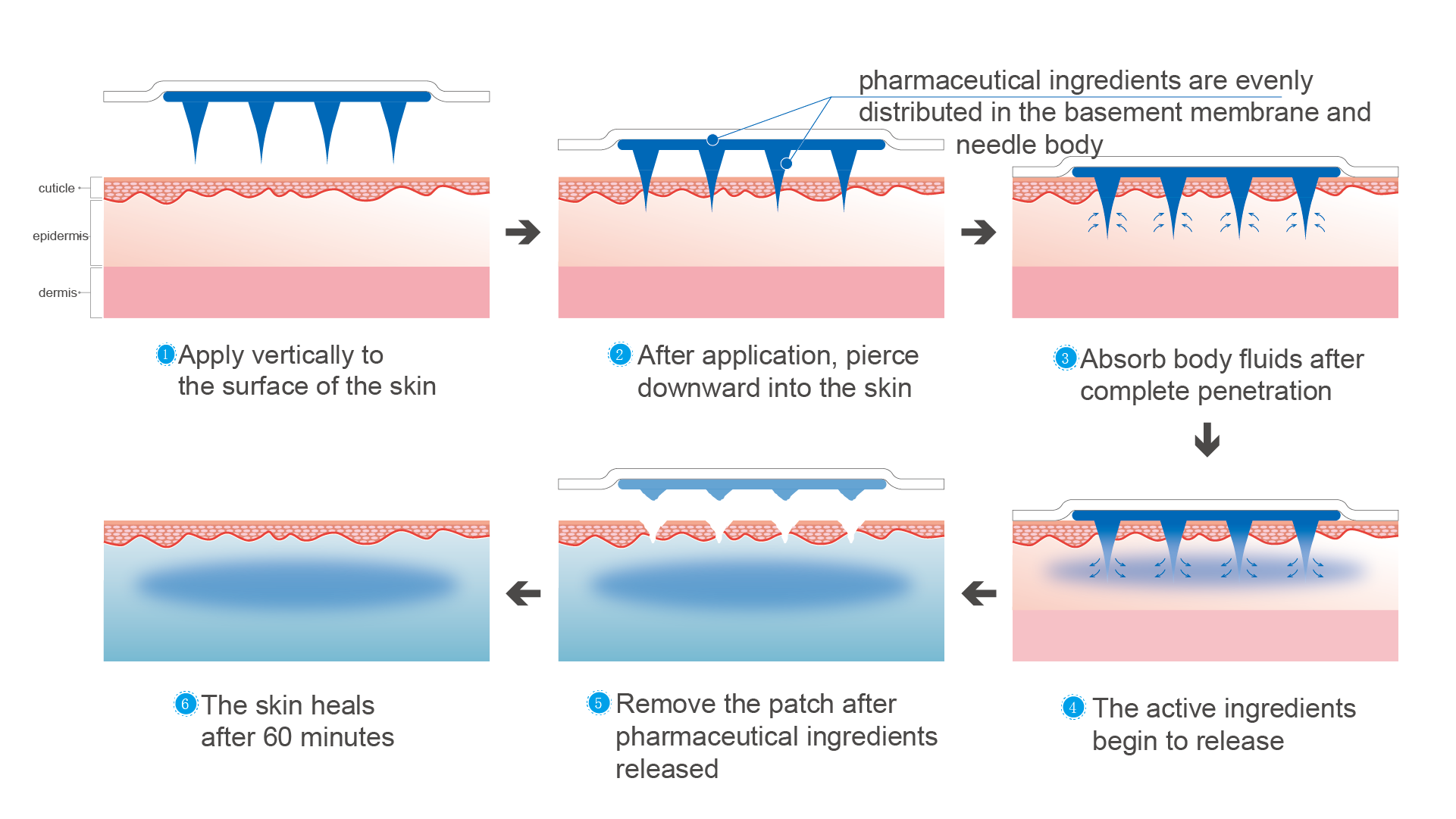
Integrated needle technology is suitable for drugs with small molecules, relatively low unit price, and high requirements in drug dose, and it needs long application time
Layered needle
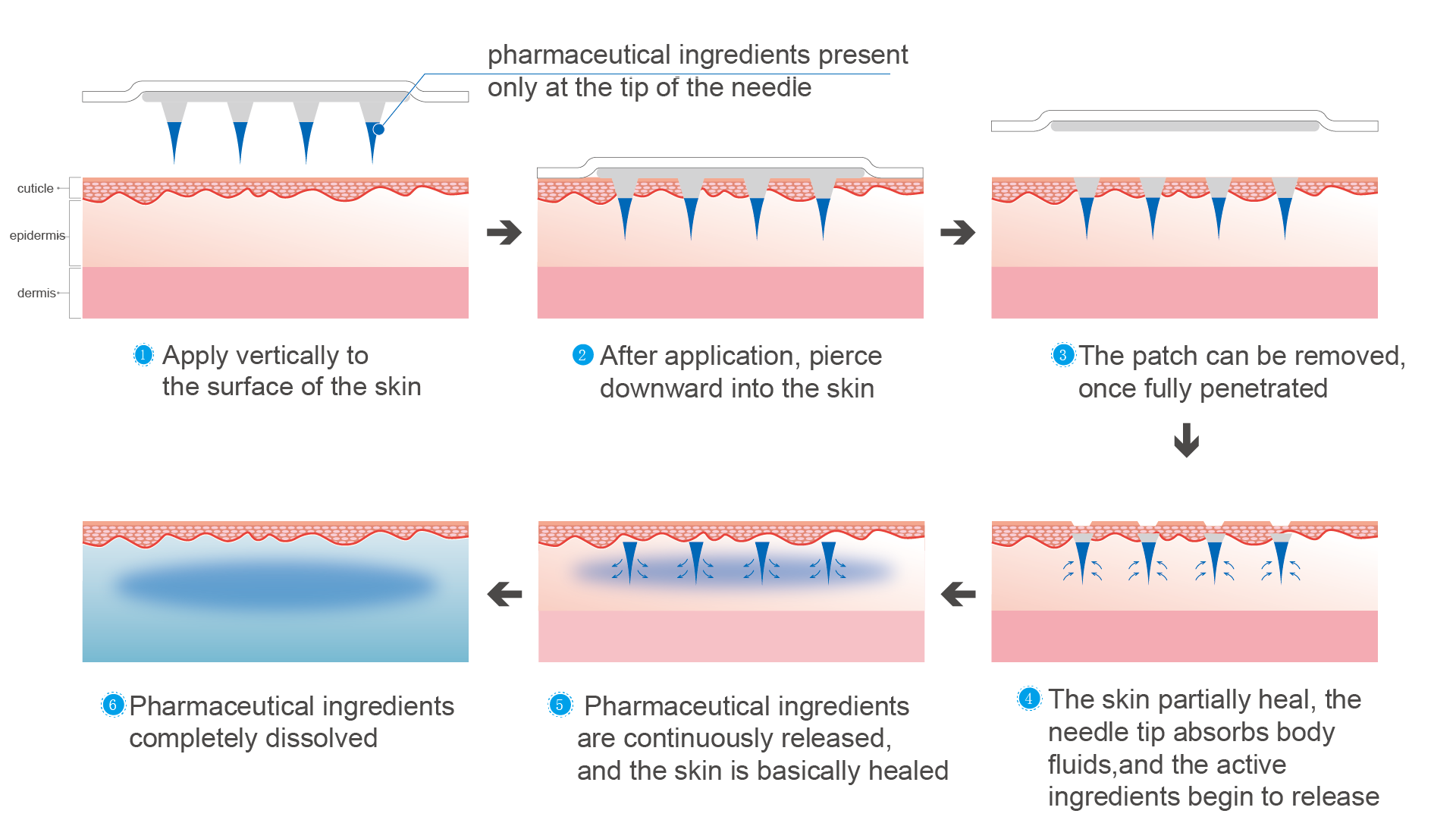
Layered needle technology is suitable for drugs with large molecules, high unit price, and low requirements in drug dose. Its application time is short, and the delivery is completed once the tip is dissolved.
Layered Needle Technology Video Demo


